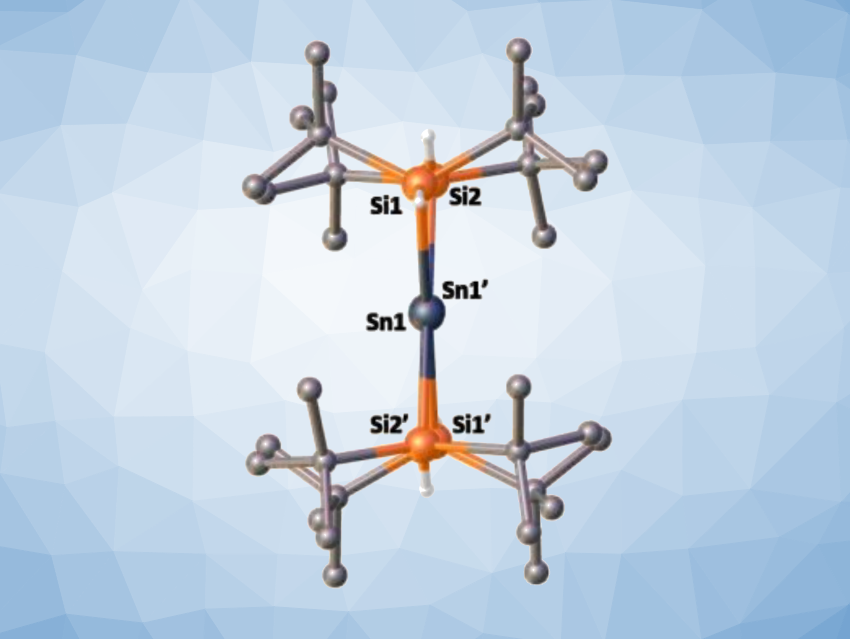
Alkenes are an important class of compounds widely used in organic synthesis. Less is known about the “heavy” analogues of alkenes, such as distannenes, which have a Sn=Sn double bond. In contrast to the planar structure of alkenes, all previously reported distannenes have a distorted structure, with either a trans-bent and/or twisted geometry around the Sn=Sn double bond (pictured below).
Dmitry Bravo-Zhivotovskii, Yitzhak Apeloig, Technion – Israel Institute of Technology, Haifa, and colleagues have synthesized the first planar non-twisted distannene, (tBu2HSi)2Sn=Sn(SiHtBu2), by mild thermolysis of (tBu2HSi)3SnH (tris(di-tert-butyl-hydridosilyl)stannane) at 70 °C in hexane. The X-ray crystallographic structure of the prepared distannene showed a planar geometry around both Sn atoms, a non-twisted Sn=Sn bond (pictured below), and the shortest known Sn=Sn bond among acyclic distannenes with a length of 2.599 Å.
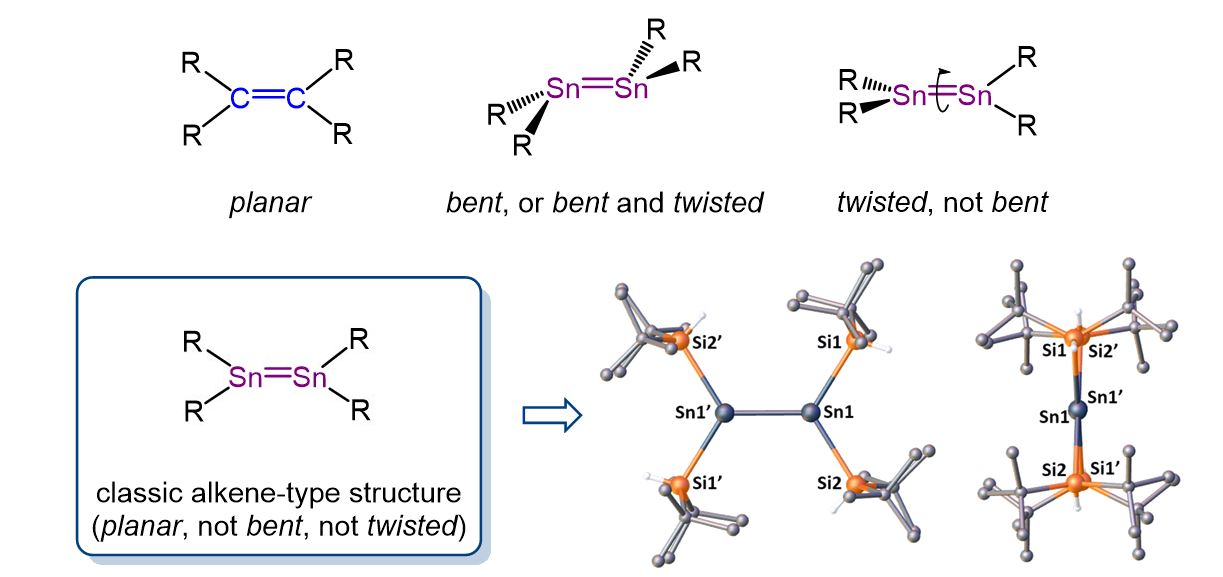
This unique combination of structural properties closely resembles the structure of alkenes. The distannene exhibits reactivity that is characteristic for a Sn=Sn double bond. For example, reactions with CCl4 or 2,3-dimethylbuta-1,3-diene produce a 1,2-dichlorodistannane or a [2+4] cycloadduct, respectively. The work contributes to the understanding of the structural, spectroscopic, and bonding differences within doubly-bonded compounds of group 14 elements.
- The First Planar, Not Twisted, Distannene – A Structural Alkene Analog. Synthesis, Isolation and X‐ray Crystallography Characterization,
Roman Bashkurov, Natalia Fridman, Dmitry Bravo-Zhivotovskii, Yitzhak Apeloig,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202302678