Aromaticity is a well-known concept in organic chemistry. However, the famous Hückel rule is not limited to carbon-based systems, but generally states that planar, cyclic, conjugated systems with (4n + 2) π electrons (n = 0, 1, 2, …) are aromatic. This can also be achieved in inorganic compounds. Still, π-aromaticity is rare in inorganic chemistry, and thus, such systems are interesting research targets. Inorganic 2π-aromatic systems, for example, are rare, and existing examples of this type of molecule are mostly charged species.
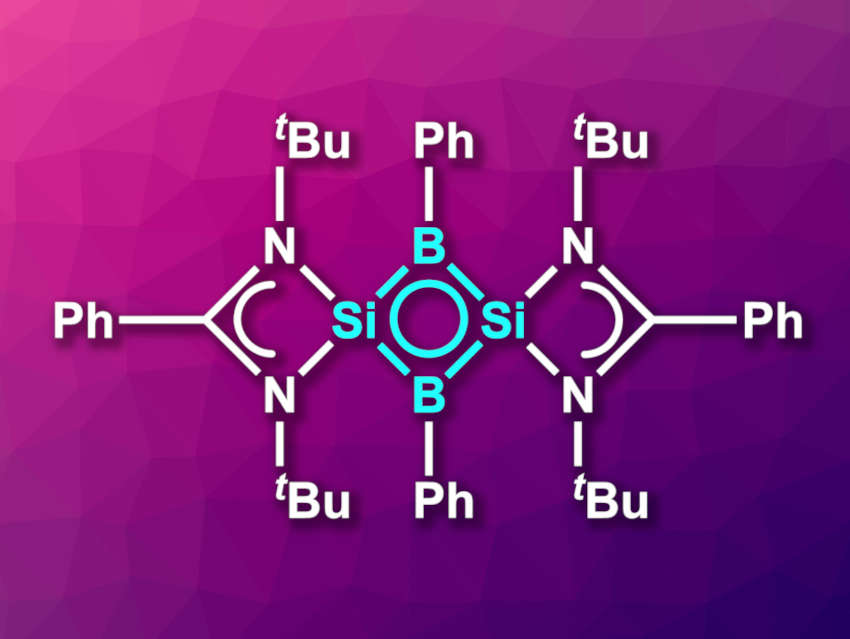
Dietmar Stalke, Herbert W. Roesky, University of Göttingen, Germany, Pattiyil Parameswaran, National Institute of Technology Calicut, Kozhikode, India, and colleagues have synthesized the first neutral inorganic four-membered 2π-aromatic compound. The compound (pictured) features an Si2B2 ring with a π-delocalized system. The team started their synthesis from an amidinato-silylene of the type LSi–R (L = amidinato ligand, R = 3,5-diphenyl-1H-pyrazolyl). This precursor was reacted with dichlorophenylborane in tetrahydrofuran (THF), starting at −78 °C and then warmed to room temperature. This was followed by a reaction with KC8 to obtain the desired product. The compound was obtained in crystalline form in a yield of 42 %.
The product was characterized using X-ray diffraction, 1H, 11B, 13C, 29Si, and 15N NMR spectroscopy, and UV/Vis spectroscopy, and its electronic structure was investigated using density functional theory (DFT) calculations. The team found that the Si2B2 ring is planar and has nearly equal Si–B bond distances. Calculations showed that the highest occupied molecular orbital (HOMO) is delocalized over the entire Si2B2 ring. Overall, the molecule can be considered a neutral, inorganic analogue of a cyclobutenyl dication.
- A Neutral Planar Four-Membered Si2B2 2π-Aromatic Ring,
Saroj Kumar Kushvaha, Paula Kallenbach, Shahnaz S. Rohman, Madhusudan K. Pandey, Zohreh Hendi, Franziska Rüttger, Regine Herbst-Irmer, Dietmar Stalke, Pattiyil Parameswaran, Herbert W. Roesky,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c09131