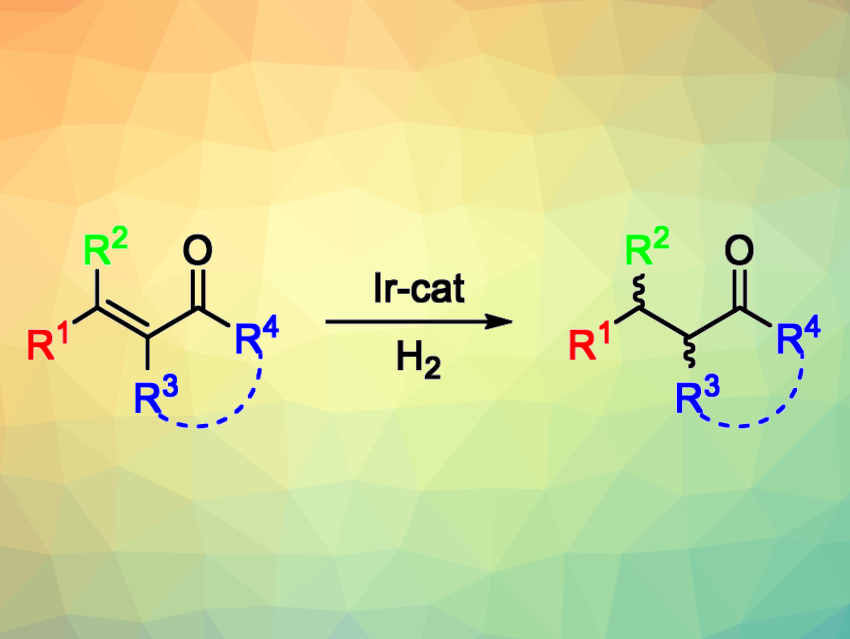
The catalytic asymmetric hydrogenation of olefins is useful for the synthesis of chiral compounds. This approach is used, e.g., in the pharmaceutical industry. However, there are still some substrates for which this type of reaction is still challenging to achieve. For example, the asymmetric hydrogenation of tetrasubstituted olefins, which generates two stereocenters, is both potentially useful and particularly difficult. Tetrasubstituted acyclic enones, for example, have remained challenging substrates so far due to the need to selectively reduce the olefin and not the carbonyl group, as well as the possibility of double bond E/Z isomerization under the reaction conditions.
Maria Besora, Montserrat Diéguez, Universitat Rovira i Virgili, Tarragona, Spain, and colleagues have developed a class of air-stable Ir-P,N catalysts that promote the asymmetric hydrogenation of a wide range of tetrasubstituted acyclic enones with high yields and enantioselectivities (general reaction pictured above). The team prepared aminophosphine-oxazoline catalyst precursors (pictured below) in three steps from commercially available amino alcohols. They found that catalyst precursors V and VI could both be used for the asymmetric hydrogenation of (E)-3-methyl-4-phenylpent-3-en-2-one, which was used as a model substrate.

The model reactions were performed under 100 bar H2 at room temperature, using CH2Cl2 as the solvent. The desired product was obtained in quantitative yield with high diastereo- and enantioselectivity within 5 min. The researchers evaluated the substrate scope and found that a wide range of electronically and sterically diverse acyclic tetrasubstituted enones (including exocyclic ones) can be transformed successfully—including some precursors of natural products and potential drugs.
- Unlocking the Asymmetric Hydrogenation of Tetrasubstituted Acyclic Enones,
Jorge Faiges, Maria Biosca, Miquel A. Pericàs, Maria Besora, Oscar Pàmies, Montserrat Diéguez,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202315872