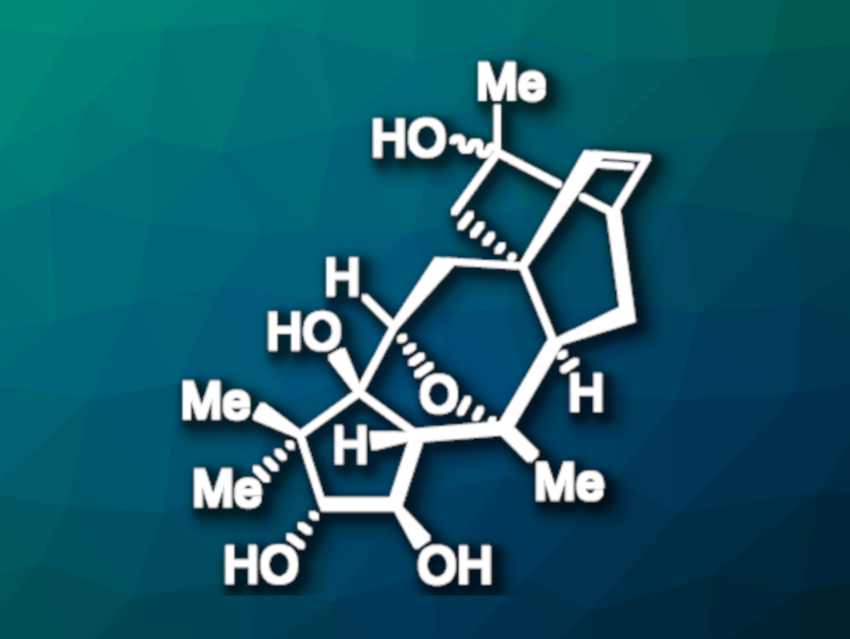
Rhodomollins A and B (pictured, rhodomollin A: α-OH, rhodomollin B: β-OH) are diterpenoid natural products first isolated from the heath family of plants (Ericaceae). The compounds belong to the grayanoid class of diterpenoids and have an oxa-bicyclo[3.2.1] core. Their complex structures make them interesting targets for total synthesis.
Ming Yang, Lanzhou University, China, and colleagues have performed the first asymmetric total syntheses of rhodomollins A and B, using a convergent strategy. The team prepared the A-ring fragment starting from an enantioenriched cyclopentanone, which was converted to a conjugated diene, dihydroxylated, stannylated, and protected with triethylsilyl chloride.
The D/E ring fragment was synthesized starting from a functionalized cyclohexene, which underwent a palladium-catalyzed carbonylation and an allylic oxidation before conversion to a diene. This diene intermediate was reacted with methyl vinyl ketone in a Diels–Alder reaction, followed by deprotection to give a cycloadduct as a single diastereomer. The resulting intermediate was subjected to an epimerization and several functional group transformations to obtain the desired fragment.
The two fragments were then combined using a Stille coupling to give a conjugated diene, followed by deprotection and a cyclization to give a tetracyclic intermediate. The oxa-bicyclo[3.2.1] core of the target natural products was then created by installing a cyclic sulfate of a 1,2-diol, followed by an intramolecular SN2 substitution. The syntheses were completed by the introduction of hydroxyl and methyl groups, using a Payne/Meinwald rearrangement sequence for the adjustment of the oxidation states in the final steps.
- Total Syntheses of Rhodomollins A and B,
Weizhao Zhao, Duo Zhang, Yuran Wang, Ming Yang,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c12249