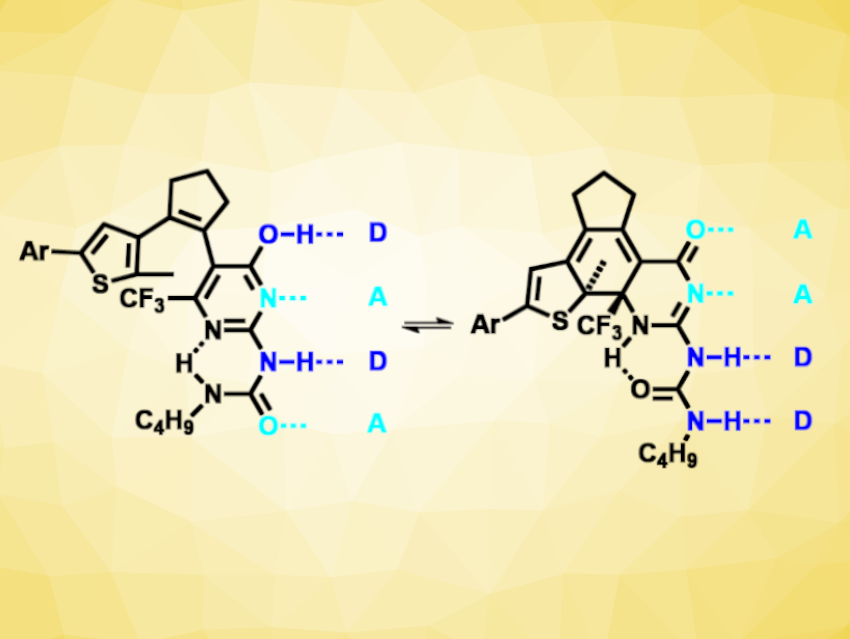
Hydrogen bonds are non-covalent interactions that Nature uses, e.g., to store and copy information in nucleic acids. More generally, hydrogen bonds can be important for molecular recognition and self-assembly. Combinations of donor (D) and acceptor (A) building blocks can be used form arrays with multiple hydrogen bonds. These arrays can then be used, for example, in supramolecular polymers or other materials. If the hydrogen-bond arrays were switchable, they could be used to build responsive materials.
Christoph Bannwarth, RWTH Aachen University, Germany, Stefan Hecht, RWTH Aachen University, DWI Leibniz Institute for Interactive Materials, Aachen, Germany, and Humboldt University, Berlin, Germany, and colleagues have developed a a photoswitchable quadruple hydrogen-bonding motif (pictured) that can be converted between two different donor/acceptor patterns (i.e., DADA and AADD). The team used a diarylethene with a ureidopyrimidin-4-ol unit, which can undergo a photochemical ring closure under 310 nm UV light and a ring opening under 490 nm visible light. These reactions cause a change in aromaticity, which then influences an enol–keto tautomerization in the compound and leads to a change in the donor/acceptor pattern.
The photoswitching is near-quantitative and reversible, and the isomers show good thermal stability. The change in donor/acceptor patterns causes a difference in dimerization constants between the two forms by over three orders of magnitude. Thus, the team’s approach could be useful for the optical control of association processes, for example, in supramolecular polymers.
- Photoswitchable Quadruple Hydrogen-Bonding Motif,
Bohan Tang, Mike Pauls, Christoph Bannwarth, Stefan Hecht,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c10401